


Other studies have emphasized the major contributions of hydrogen peroxide production via chemical interactions with the gas ozone, O 3, and a process called cavitation, when vapor bubbles arise in low-pressure areas within accelerated liquids. The new findings could help settle some of the debate that has ensued in the scientific community since the Stanford researchers initially announced their novel detection of hydrogen peroxide in water microdroplets three years ago.
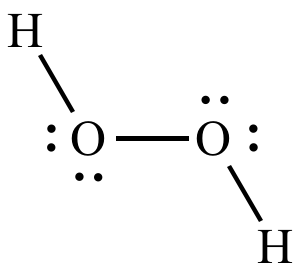
Comparing the post-reaction mix of water and hydrogen peroxide fluid from the treated and untreated channels showed the signal of 18O in the former, implicating the solid as the source of the oxygen in the hydroxyl radicals and ultimately in hydrogen peroxide. These treated channels contained a heavier isotope or version of oxygen, dubbed oxygen-18 or 18O. To gauge if the extra oxygen atom in the hydrogen peroxide (H 2O 2) came from a reaction with the glass or within the water (H 2O) itself, the researchers treated the glass lining of some microfluidic channels. Additional experiments elaborated that the hydrogen peroxide formed quickly, within a matter of seconds, at the boundary between the water and the solid. An experiment showed the presence of the harsh chemical in the glass microfluidic channel, but not in a bulk sample of water also containing the dye. The researchers perfused the water with a fluorescent dye that glows in the presence of hydrogen peroxide. The channels formed an airtight water-solid boundary. On the origins of the hydrogen peroxideįor the study, the researchers built a glass apparatus with microscopic channels in it where water could be forcibly injected. 1 in the Proceedings of the National Academy of Sciences (PNAS).

Zare led this work, collaborating with researchers from two universities in China, Jianghan University and Wuhan University, as well as the Chinese Academy of Sciences. “Furthermore, it appears that contact electrification yielding hydrogen peroxide is a universal phenomenon at water-solid interfaces.” “We have a real understanding now that we didn’t have before about what is causing this hydrogen peroxide formation to happen,” said study senior author Richard Zare, the Marguerite Blake Wilbur Professor in Natural Science and a professor of chemistry in the Stanford School of Humanities and Sciences. Those additional findings suggest that water can transform into small amounts of reactive oxygen species, such as hydrogen peroxide, wherever microdroplets naturally form, including in fogs, mists, and raindrops, bolstering results from a related 2020 study. The new study further demonstrated that this process occurs in humid environments when water touches particles of soil as well as fine particles in the atmosphere. Pairs of these species known as hydroxyl radicals, and which have the chemical formula OH, can then combine to form hydrogen peroxide, H 2O 2, in minuscule but detectable quantities. Electric charge jumps between the two materials, liquid and solid, producing unstable molecular fragments called reactive oxygen species. The latest study has revealed that when sprayed microdroplets of water strike a solid surface, a phenomenon known as contact electrification happens. Researchers have since aimed to flesh out how the newfound reaction occurs, as well as exploring potential applications, such as eco-friendlier cleaning methods. (Image credit: Getty Images)īack in 2019, Stanford University researchers and colleagues revealed the surprising discovery that hydrogen peroxide – a caustic substance used for disinfecting surfaces and bleaching hair – spontaneously forms in microscopic droplets of ordinary, benign water. Following up on work that found that microdroplets of water can spontaneously form hydrogen peroxide, researchers have determined this is the result of the water contacting a solid surface and producing electric charge.


 0 kommentar(er)
0 kommentar(er)
